Want to Subscribe?
Read Corporate India and add to your Business Intelligence

![]() Unlock Unlimited Access
Unlock Unlimited Access

Published: August 15, 2024
Updated: August 15, 2024
This fortnight we have picked Syngene International, a Bengaluru-headquartered integrated research, development and manufacturing services organisation, serving the global pharmaceutical, biotechnology, nutrition, animal health, consumer goods, speciality chemical and Biocon sectors. The company manages a pool of over 5,000 scientists, including 500 Ph.Ds and 2,750 scientists with a Master’s degree.
The company owns state-of-the-art research facilities spread over 9,00,000 sq ft, certified by major regulatory bodies. It serves mainly global innovator pharma/chemical companies, offering integrated scientific services from early discovery to commercial supply. The company is known for establishing long-lasting relationships with its customers and this has emerged as its key strength. Some of the strategic collaborations are with Bristol Myers Squibb, Baxter, Endo Pharmaceuticals and Abbott Nutrition.
Syngene serves leading players in pharma, biotechnology and other related fields which outsource some or a substantial part of their business in the product development lifecycle, and operates via ‘full time equipment’ (FTE) and ‘fee for services’ (FFS) models.
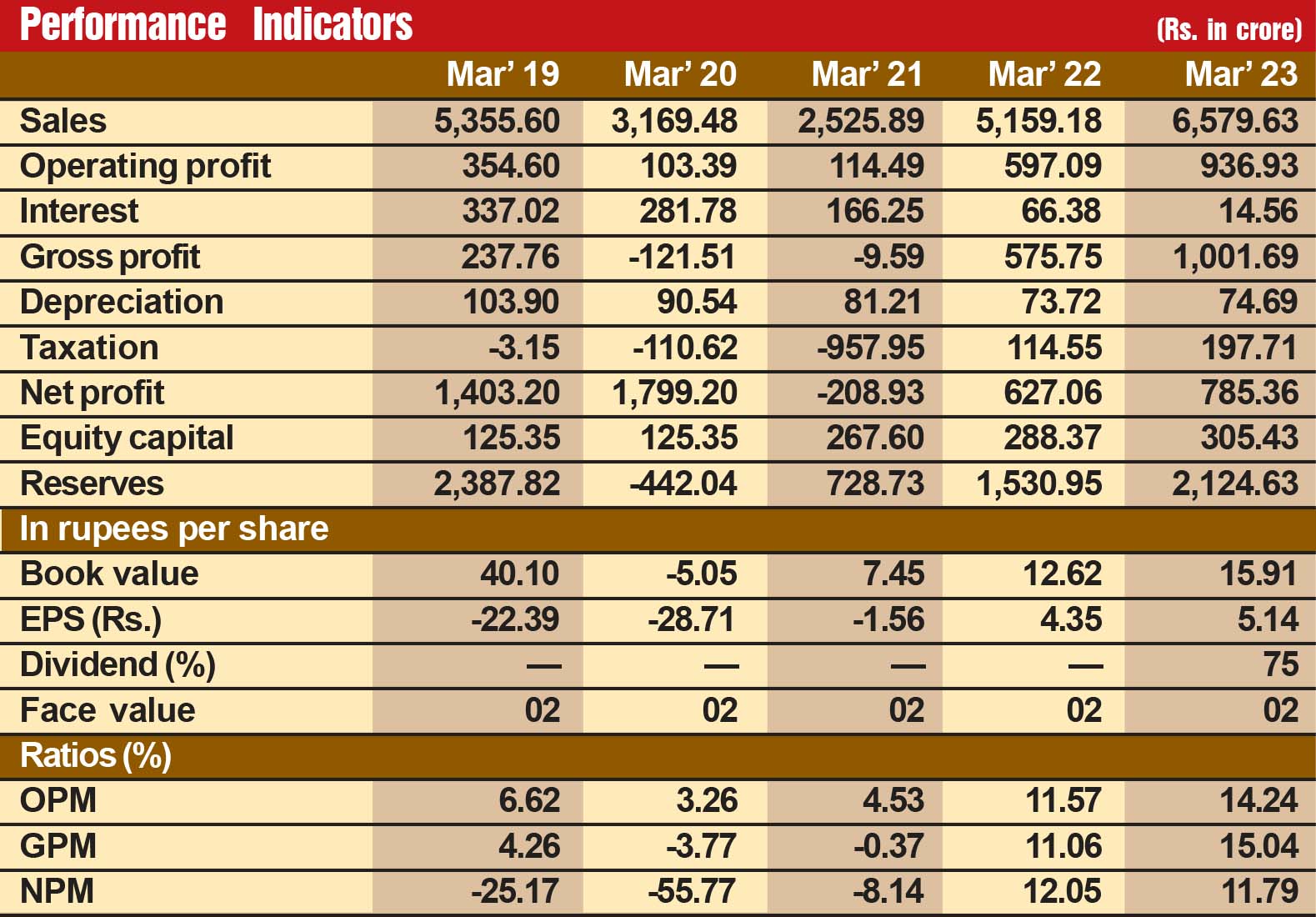
The company is steadily growing in its financial performance. During the last seven years, its sales turnover has expanded from Rs 1,423 crore in fiscal 2018 to Rs 3,489 crore in fiscal 2024, with operating profit advancing from Rs 474 crore to Rs 1,047 crore and the profit at net level inching up from Rs 305 crore to Rs 510 crore. Its financial position is very strong, with reserves at the end of March 2024 standing at Rs 3,856 crore – over nine times its equity capital of Rs 402 crore. During this 7-year period, the company’s EPS has steadily moved up from Rs 7.64 to Rs 12.69. This clearly suggests efficient cost management and enhanced operational efficiency.
But we have not picked Syngene as the Fortune Scrip for its past laurels. We are of the strong opinion that future prospects for the company are all the more promising. Consider:
The company's future prospects are all the more exciting as it has recently undertaken several initiatives to expand its business through expanding existing relationships, developing new partnerships and making investments in manufacturing capacity. Key among them has been the extension of Syngene's research collaboration with leading biotech company Amgen Inc until 2026. Its scope includes Syngene providing integrated drug chemistry and biology, peptide chemistry, antibody and protein reagents, pharmacokinetics, and drug metabolism and pharmaceutical development, in addition to operating the existing Syngene-Amgen R&D centre. Under the new contract, Syngene will also build and operate a dedicated laboratory which will enable R&D project deceleration.
Another notable contract the company signed recently was with leading animal health company Zoetis. The contract signed in July 2022 is for a period of 10 years and is for manufacturing the drug substance for Librela (bedinwetmab), a first-in-class monoclonal antibody used for treating osteoarthritis in dogs. Initially centred on Librela, this agreement paves the way for developing and manufacturing other molecules in the coming years. Thanks to this contract, Syngene will be able to move from a CRO to a CRAMS (Contract Research and Manufacturing Services) company. It has got an advantage of scale as it has already an established relationship with clients from research to, now, manufacturing, and this transition may prove to be the next trigger of growth. The partnership aims to provide innovative research and development services to Zoetis, focusing on the animal health market. Working with Zoetis enables Syngene to gain access to Zoetis's domain expertise in animal health research.

January 31, 2026 - Second Issue

Industry Review

Want to Subscribe?
Read Corporate India and add to your Business Intelligence

![]() Unlock Unlimited Access
Unlock Unlimited Access
Lighter Vein

Popular Stories
Archives
